Melatonin, N-acetyl-5-methoxytrptophan, is an indoleamine; an amine derivative. Amine derivitives are relatively small molecules, produced from enzymatic processing of the amino acid tryptophan (Hadley & Levine, 2007), as described in Assignment 1 (refer to biosynthesis pathway).

A comparison of the structure of our hormone amongst three different species was to be completed using BLAST. However, because melatonin is not a peptide, it has no amino acid sequence to compare. Instead, the protein sequence for a related protein structure, the melatonin receptor 1A, was compared.
Structure of Melatonin Receptor 1A
The mammalian melatonin 1A receptor is a high-affintiy, G-protein-coupled receptor expressed in the hypothalamic suprachiasmatic nucleus and the pars tuberalis of the hypophysis (Reppert, 1997).
The 1A receptor is similar to all other G-protein-coupled receptor superfamily members, however they lack some highly conserved sequence motifs (eg. D/ERY motif). This integral membrane protein has seven transmembrane alpha-helical domains, connected by 3 intracellular and 3 extracellular loops, making it similar in structure to the protein rhodopsin (below). The amino terminus is on the extracellular side of the membrane, while the carboxyl terminus is located on the intracellular side of the membrane (Barrett et al., 2003).
 Figure 1: An overlay of the 3D structure of the human melatonin receptors MT1 (as discussed above) and MT2, as well as rhodopsin (another similar G-protein coupled receptor); the active binding sites of these receptors are shown in the bottom two panels (from: http://model.nmr.ru/img/projects/gpcr/MTR_structure.en.png)
Figure 1: An overlay of the 3D structure of the human melatonin receptors MT1 (as discussed above) and MT2, as well as rhodopsin (another similar G-protein coupled receptor); the active binding sites of these receptors are shown in the bottom two panels (from: http://model.nmr.ru/img/projects/gpcr/MTR_structure.en.png) Figure 2: Transverse section of human MT1 receptor (from: Barret et al., 2003).
Figure 2: Transverse section of human MT1 receptor (from: Barret et al., 2003).There are several amino acid residues found on domains 3, 5, 6, and 7 which are important in the binding of melatonin. The methoxy group of melatonin proposedly interacts with the His211 residue on transmembrane domain 5. Mutation of His211 can reduce binding by up to seven-fold, depending on the substitution (Barrett et al., 2003).
 Figure 3: Orientation of melatonin in binding pocket of melatonin 1A receptor; note the interaction of the -CH3 (methoxy) group and His211. Darkened residues indicate substitutions. (from: Barrett et al., 2003).
Figure 3: Orientation of melatonin in binding pocket of melatonin 1A receptor; note the interaction of the -CH3 (methoxy) group and His211. Darkened residues indicate substitutions. (from: Barrett et al., 2003).Sequence alignment
The sequence for the melatonin 1A receptor was found for the following 3 species:
- humans
- mice
- cod fish
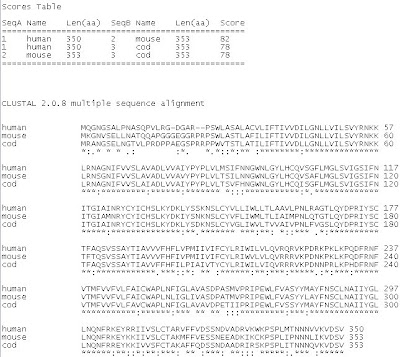 Key:
Key:* = identical residues
: = conserved substitutions
. = semi-conserved substitutions

Figure 4: Phylogram depicting relationship between humans, mice, and cod based on the protein structure of the melatonin receptor 1A (from Clustal).
It can be seen in the scores table above that the alignment of melatonin receptor 1A in humans and mice (score of 82) is more common than either humans or mice with cod (score of 78). This is also illustrated in the phylogram (refer to Figure 1 above). Humans and mice, both of which are mammals, are more closely related than either of them which cod fish. It can also be assumed from the alignment, that the structure of melatonin receptors is not highly conserved among species due to these differences.
References:
Barrett, Perry, Shaun Conway, and Peter J. Morgan. "Digging deep - structure-function relationships in the melatonin receptor family." Journal of Pineal Research. 35(2003): 221-230.
Hadley, Mac E, and Jon E. Levine. Endocrinology. 6th ed. Upper Saddle River, NJ: Pearson Prentice Hall, 2007.
Nelson, Cole S., Masayuki Ikeda, Heinrich S. Gompf, Mindi L. Robinson, Nadine K. Fuchs, Tohru Yoshioka, Kim A. Neve, and Charles N. Allen. "Regulation of Melatonin 1A Receptor Signalling and Trafficking by Asparagine-124." Molecular Endocrinology. 15(2001): 1306-1317.
Reppert, Steven M. "Melatonin Receptors: Molecular Biology of a New Famimly of G Protein-Coupled Receptors." Journal of Biological Rhythms. 12(1997): 5528-531.